Fundamentals of Refrigeration: Heat Transfer
The movement of heat is traditionally referred to as “heat transfer”. Thermodynamics is a term applied to the study of heat movement. The laws of thermodynamics are a crucial foundation for understanding heat movement.
The first law of thermodynamics states that energy can be neither created nor destroyed, merely changed.

Since heat is a form of energy, the first law of thermodynamics teaches us that to refrigerate something, we must transform or move the heat from where it is unwanted to another area where it will do no harm.
The second law of thermodynamics teaches that heat always flows from a higher temperature to a lower temperature.
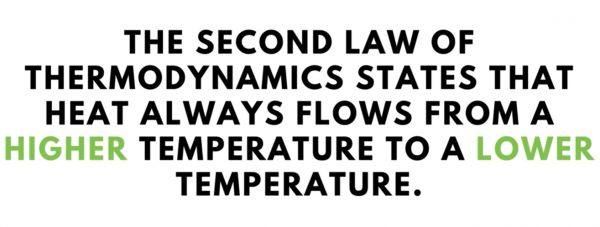
We know this intuitively because when we use a spoon to stir a hot beverage, we expect the spoon to heat up. This is the second law of thermodynamics at work. Heat is being transferred from the hot drink into the spoon because of the temperature difference.

The second law of thermodynamics parallels the law of fluid flow, which states that flow is proportional to pressure difference. Just as more pressure results in more fluid flow, a greater temperature difference will result in more heat transfer.


Leave a Reply