Fundamentals of Refrigeration: Sensible Heat
Oftentimes, heat gains and losses can be measured with a thermometer. When this is the case, the heat can be “sensed” and we call it “sensible heat”. Raising the temperature of water from 20ºF to 40ºF is an example of sensible heat.
The formula used to calculate the heat required to raise the temperature of an object is called the “sensible heat equation” and is expressed as ‘Q’ equals ‘M’, ‘C’, ‘ΔT’. Where ‘Q’ is the heat quantity measured in BTUs, ‘M’ is mass, measured in pounds, ‘C’ is the specific heat of the substance, measured in BTUs per pound ºF, and ‘ΔT’ is the temperature change measured in ºF.
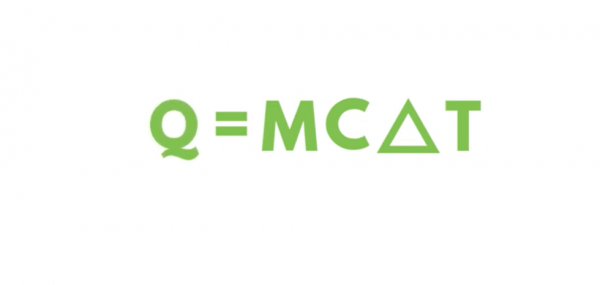
The term ‘delta’ is commonly used for differential measurements. The triangle symbol (Δ) is the character used in the Greek alphabet for the letter ‘delta’.

The sensible heat equation can be applied anytime there is a change in the temperature.
Example 1:
How many BTUs are required to change the temperature of 4 pounds of water from 30ºF to 50ºF?
To solve this problem, first identify what is known. In this problem, the mass is given as 4 pounds. The problem states that we are heating water, and the specific heat of water is 1 BTU per pound ºF. The temperature change is 20°F, which is the difference between the starting temperature, 30ºF , and the final temperature, 50ºF.
After plugging the known values into the equation, the solution is that 80 BTUs are required to raise the temperature of 4 pounds of water from 30ºF to 50ºF.
Example 2:
How many BTUs are required to change the temperature of 4 pounds of iron from 30ºF to 50ºF?
The specific heat of iron is 0.118 BTU per pound ºF. The equation will be the same, except that the value for specific heat is substantially lower.
Plugging the values into our calculator reveals that 9.44 BTUs are required to change 4 pounds of iron by 20ºF. Due to the difference in specific heat, it is much easier to heat iron than it is to heat water.
Example 3:
It takes 100 BTUs to heat 2 pounds of an unknown substance 25ºF. What is the specific heat of the substance?
The first step is to rearrange the equation to solve for ‘C’, or specific heat. Whatever is done to one side of the equation must also be done to the other side.
The new equation is ‘C’ equals ‘Q’ divided by ‘M ΔT’. Next, the given values can be plugged into the equation to determine that the unknown substance has a specific heat of 2 BTUs per pound ºF.

Leave a Reply