Fundamentals of Refrigeration: Specific Heat
Have you ever wondered why some things heat up quicker than others? Or why some substances might take several minutes to get hot, while others only take a few seconds? It is well understood that as heat is applied to a substance in a single state, the temperature of the substance will increase. Experiments have proven that some substances can be heated more easily, or with less heat added, than other substances. The term scientists and engineers use to describe this material property is “Specific Heat”.

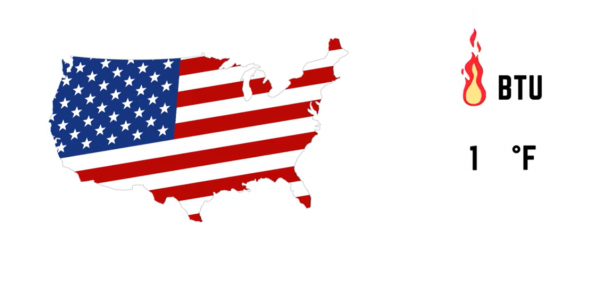
Specific heat is defined as the amount of heat required to raise the temperature of a substance by one degree. In the United States, the customary unit for heat is BTU, and for temperature it is ºF, so specific heat is the amount of BTUs required to raise one pound of a substance one ºF.
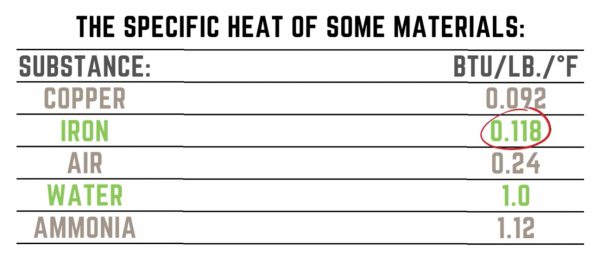
From the listed materials above, it is obvious that Iron will heat up much quicker than water because it has a much lower specific heat.
The major takeaway is that materials with a low specific heat are easy to heat or cool. On the flip side, a material with a high specific heat is difficult to heat or cool, making it an ideal insulating material.


Leave a Reply