Fundamentals of Refrigeration: Heat
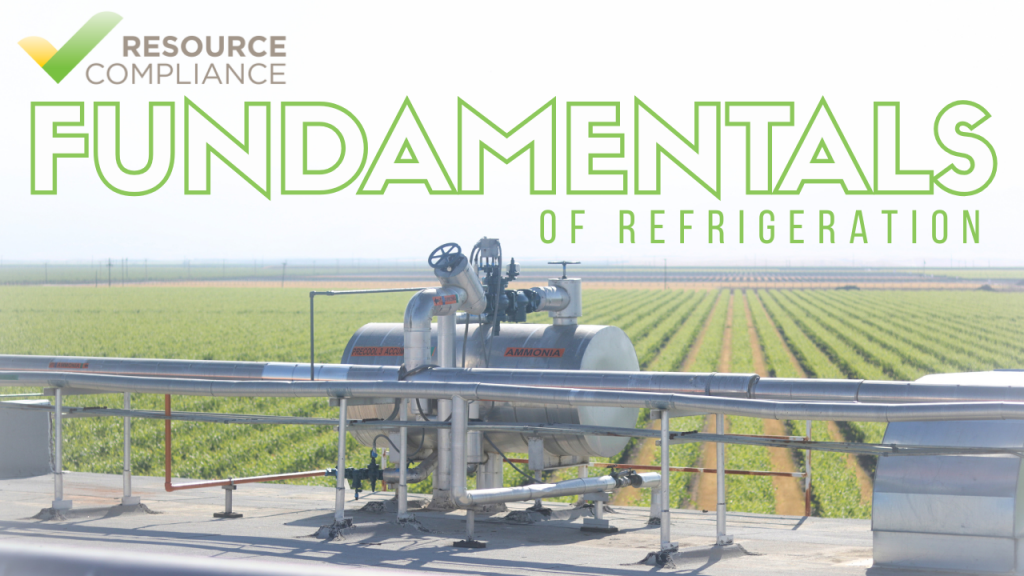
In scientific terms, “heat” is synonymous with energy and work.
To explain heat, it is best to start small. All matter is made up of molecules. Molecules that contain heat energy are in constant motion. The speed of the molecular motion is directly related to the temperature of the matter. The faster, or more intense, the motion, the higher the temperature will be.
Similarly, as molecular motion slows, the temperature drops. When all molecular motion stops, the substance is said to be at “absolute zero,” which corresponds to a temperature of 0ºR, or -459.67ºF. Thermometers measure molecular movement and convert it into familiar units such as ºC or ºF.
When the temperature of a fixed amount of matter changes, it always results in a change in heat content. However, a change in heat content does not always result in a temperature change. For example, when a pot of water is boiling, heat is added, but the temperature does not change.
In refrigeration applications, the typical unit of heat is BTU, or British Thermal Unit. “Calorie”, “joule”, and “kilowatt-hour” are other units of heat that are common in other industries or outside the United States.
One BTU is the amount of heat required to raise the temperature of one pound of water one ºF. Similarly, one “calorie” is the amount of heat required to raise the temperature of one gram of water one ºC.
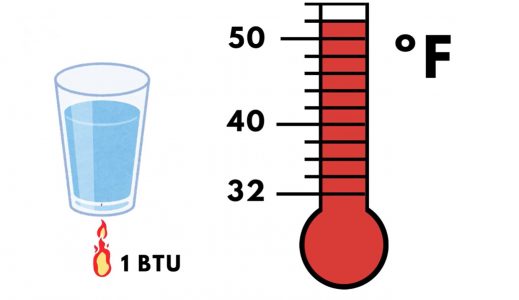
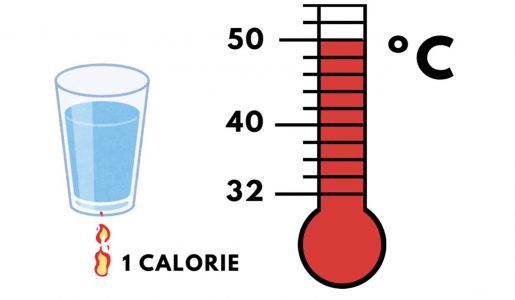
As with other units of measurement, BTUs can be converted to other units of heat. For example, one kilowatt-hour is equivalent to 3,412.14 BTUs.

Leave a Reply