Principles of Refrigeration
In order to properly identify and control hazards in an ammonia refrigeration system, it is necessary to understand how a refrigeration system operates. Without understanding the basic principles, one is likely to be confused, slow to respond, or even incorrect when identifying unsafe conditions.
What is refrigeration?
Refrigeration is a process wherein the pressure of Substance 1 is manipulated in order to control the temperature of Substance 1 for the purpose of lowering the temperature of Substance 2.
Practically speaking, Substance 1 is a refrigerant, with ammonia being the most commonly used in industrial refrigeration systems, whereas Substance 2 can be anything we desire to cool: air, water, beef, grapes, wine, glycol, apples, chicken, beer, fish, lettuce, ice cream, etc.
When studying the field of thermodynamics, we learn that when the liquid and vapor states of a substance coexist within a container, as we lower the pressure within the container, the temperature of the substance is simultaneously lowered. This condition of coexisting liquid and vapor within a container is often referred to as a saturated condition. The pressure-temperature relationship at saturation is extremely valuable since we can easily modulate the pressure of a vapor using a compressor. This means we can use a compressor to lower the pressure of a refrigerant to a level that corresponds to the temperature that will achieve the cooling effect that we desire.
Imagine that we have container with coexisting liquid and vapor ammonia. A pipe protrudes from the top of the container and is connected to the inlet (suction) of a compressor. As the compressor is turned on, ammonia vapor is drawn from the container, thus reducing the pressure and temperature within the container.
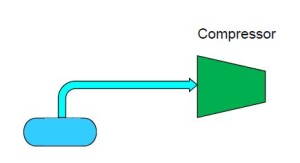
Now expand this description and imagine that we connect another pipe from the bottom of the container to a heat exchanger designed to cool air. Liquid ammonia is allowed to flow through the pipe into the heat exchanger which is lowering the temperature of warm air as it passes over the heat exchanger containing the ammonia. As the warm air is cooled, heat is transferred into the saturated ammonia liquid, causing it to form a liquid/vapor mixture and eventually 100% vapor. In one sense, this description already demonstrates how refrigeration works, but we have a serious problem…ammonia is escaping from the discharge of our compressor and also from the outlet of our heat exchanger.
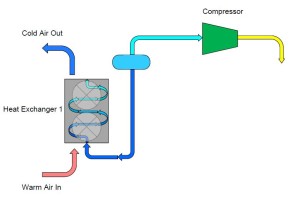
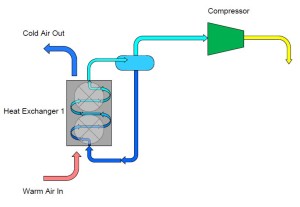
To remedy this problem we can add a third pipe to the container which connects the outlet of the heat exchanger back to the container. In doing this, we have “collected” the ammonia which evaporated while the warm air passed over it in the heat exchanger. This vapor can then continue on to the compressor which is controlling our pressure and temperature.
On the outlet of the compressor we are now going to extend a pipe which connects to a second heat exchanger. This heat exchanger is similar to the one described earlier except that instead of warm air passing over the heat exchanger, we are now going to have cool air pass over the heat exchanger. The effect of this step is to cause the heat that was added to the ammonia inside the first heat exchanger combined with the heat that was gained during the compression process to be rejected into the cool air. As the ammonia vapor is cooled it will eventually reach its saturation condition and begin to form a liquid-vapor mixture and finally fully condense into a liquid. The resultant condition of the ammonia at the outlet of the second heat exchanger is a high pressure, saturated liquid.
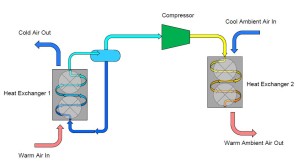
To complete our cycle, we now install a final pipe connecting the outlet of our second heat exchanger to the original container. This will replenish the container with liquid ammonia that evaporates in the first heat exchanger. However, we do not want to introduce high pressure/temperature liquid into the container that we desire to collect and supply low temperature/pressure ammonia. To remedy this, we install an obstruction within the pipe which creates a pressure reduction across the reduction. Typically, this obstruction is in the form of a valve, known as an expansion valve.
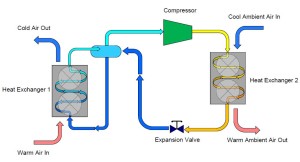
This simple explanation of a refrigeration system began with a container of saturated ammonia. To simplify our cycle even further, we can actually eliminate the original container from our cycle and connect the outlet of the expansion valve directly to our first heat exchanger. Similarly, the outlet of the first heat exchanger can be piped directly to the compressor.
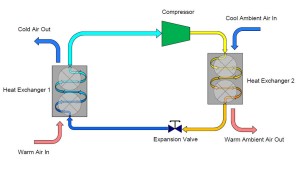
There you have it. Refrigeration is an understandable concept when broken down into its individual steps.

Leave a Reply